Personalized medicine is also called precision medicine. At Sanus we empower personalization of therapy through advanced biomarkers or biological measurables, such as genes, proteins, or activity metrics of cellular behavior. By empowering patients we create a repository of living personal medical data. The connection between B2C and B2C markets is at the core of our B2B2C business. In this section we utilize the language of the biotech industry, and present some of our own terms. Specifically, we empower an advanced form of decentralization or distributed clinical trials (DCTs), with many biomarkers (biomarker-rich or BMR), with outcomes-based contracts (OBCs) delivered through a cloud platform or software-as-service (SaaS). The resulting business platform can be described as SaaS-DCT-BMR-OBC and offers unique advantages for the industry. Our tools can help to cure the endemic problem of dismal return on investment (ROI) within the drug development industry which has historically languished with ROIs below that of utility providers.
By providing value to drug developers and the biotech industry we are able to reverse the flow of funds, specifically, we create a new model where drug developers pay patients for access to the essence of their medical case. Traditionally, this value is not shared with the patients at all. We can achieve substantial revenue sharing for the patient while providing great value to the industry. First, we address a critical problem of patient and clinical test site enrollment and retainment which together account for 56% of clinical trial costs. We achieve this by continuous engagement of the patient through our First Opinion™ portal. Second, we facilitate the development of companion diagnostic tests targeting critical biomarkers which can predict patient response to the therapy. Biomarkers have had great impact on the bottom line, increasing the rate of approval of oncology drugs 3 – 5x or greater. In calculations carried out for breast cancer, the overall impact of biomarkers accounting for all phases of clinical trials, the overall impact on the success of the drug development program’s approval chances were 16x higher! This is not surprising because therapy without biomarkers, whether single-agent or combination therapy for cancer generally has a 30-40% objective response rate.
With biomarkers, effectiveness can increase to as high as 80-90%. Finally, outcomes-based medicine that is expected to revolutionize healthcare needs to be financially sustainable. Rather than reimbursement for drugs regardless of impact, paying in proportion to the impact on patients can improve the bottom line for corporations. This situation can be compared to the initial reluctance of movie makers to accept the movie rentals business model. Eventually, they understood this created a new market that did not come at the expense of an existing one — going out to see a movie. Similarly, we need to reward drug makers for improved effectiveness of therapy, not just the size of their customer base.
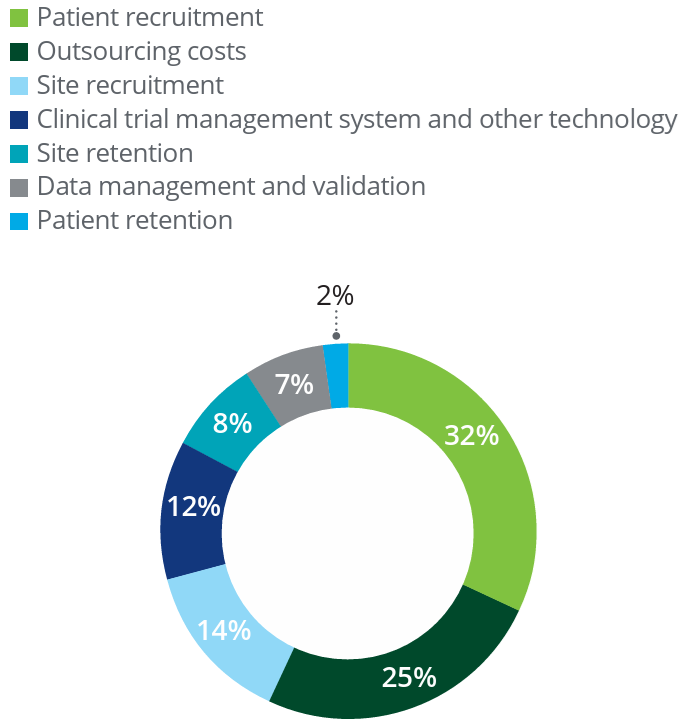
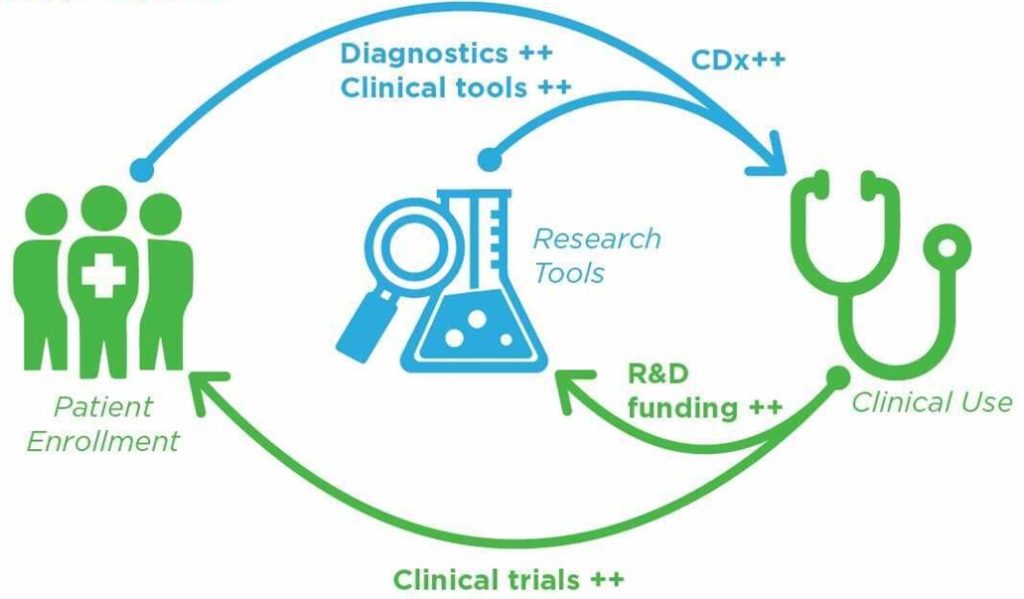
As the graphic above illustrates, there is a virtuous cycle between adopting new tools (and drugs) in research and proceeding to clinical use. The keys are healthcare impact and the ability to enroll more patients, both of which Sanus can deliver. Identifying and enrolling suitable patients in clinical trials is a serious bottleneck in the clinical trial process, as identified by Deloitte research in a 2019 study and reproduced below. In fact, between enrollment and retention of patients and clinical sites (mostly hospitals, but also some CROs) which accounts for 56% of overall cost of clinical trials.
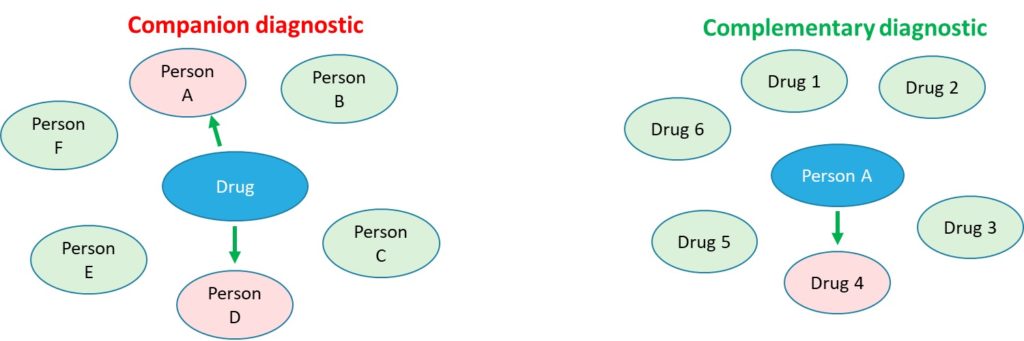
Sanus can not only align interests of the industry with those of the patient by reimbursement that is effectively graded by its effectiveness but can also provide an important bridge between the patient and industry. As a champion of the patient, we choose to adopt industry terms like precision medicine and companion diagnostics to help the patient obtain the most value (revenue sharing) for their data. Regarding the term companion diagnostics, we also apply a more patient-centered version called complementary diagnostics. This distinction has actually been made by the FDA. Complementary diagnostics is more comprehensive and puts the patient, not a drug at the center of therapy. While companion diagnostics is able to answer the question about suitability of the patient therapy (one drug or in combination), a negative answer leaves the patient stranded. Complementary diagnostics is able to evaluate all potential therapies for the patient and predict the most suitable therapy.
Precision or personalized medicine, companion or complementary diagnostics are subtle distinctions that we navigate in order to maximize monetization of medical data for the patient. By catering to the drug development industry with patient and hospital enrollment, the high quality of biomarker data in our Medical Data Ocean and associated analytical tools, we empower drug developers to perform Virtual Drug Screening (VDS) that allows them to compare biological responses of their drug to many others, including in clinical conditions saving time and money. VDS allows drug developers to obtain 50-80% of the information they need for 5% of the cost and 5% of the time.

